Entries in Politics and government (199)
H1N1v swine flu jumps species barrier again, infects Iowa housecat
A Reuters story from today underscores just how resilient and unpredictable swine flu really is. A housecat in Iowa was feeling kinda poopy, as were two human members of the household, owned (as everyone knows) by the housecat.
A vet swabbed the housecat and sent the sample off to be tested. Sure enough, the sample came back positive for H1N1v. This marks the first time a cat has been diagnosed with H1N1.
 But felines catching pandemic or potentially pandemic strains of flu is not new. Recall the Bangkok Zoo tigers -- almost 60 in total -- who had to be euthanized because they ate chickens tainted with H5N1 bird flu. Also, in Indonesia, it is estimated that 20% of all cats contain antibodies to H5N1, because they have feasted or nibbled on poultry and wild birds infected with bird flu. H5N1 is still endemic to Indonesia, and a couple of years ago, the Indonesian Army was called upon to actually swab housecats to check for H5N1 antibodies (photo at left).
But felines catching pandemic or potentially pandemic strains of flu is not new. Recall the Bangkok Zoo tigers -- almost 60 in total -- who had to be euthanized because they ate chickens tainted with H5N1 bird flu. Also, in Indonesia, it is estimated that 20% of all cats contain antibodies to H5N1, because they have feasted or nibbled on poultry and wild birds infected with bird flu. H5N1 is still endemic to Indonesia, and a couple of years ago, the Indonesian Army was called upon to actually swab housecats to check for H5N1 antibodies (photo at left).
Bird flu just flared up again in Vietnam, in an area (Diem Bien province) where it had not been seen in several months. Some 3,000 birds were culled to try and prevent its spread, even though bird flu needs to fire its press agent and hire a new one.
Should we be surprised by this latest development? I am not sure. Perhaps this has more to do with bird flu's affinity for the hunter (cats) and, as my recent blog said, sometimes swine flu runs home to Momma, Momma being the 1/3 avian origin of this unique hybrid pandemic virus. So I am not surprised that H1N1v would jump the species barrier again. Nothing about this virus surprises me anymore.
Dogs are another matter. H3N8 equine influenza crossed the species barrier to dogs in Florida sometime in the past 10 years, but there are no signs that dogs have caught swine flu. There are signs, however, that dogs have caught H5N1 bird flu. In my blog, "Beware of Dog" gains new meaning, I talk about the Dutch experiment regarding H5N1. Dogs can, indeed, catch avian flu. So it would not be surprising, then, if Fido or Buster caught swine flu.
If you read the aforementioned blog, from 2007, it indicates H5N1 droplet nuclei were present and active in dogs' nasal secretions. There are some instances of H3N8 canine influenza in this country right now. Is dual infection likely or unlikely? A lot of people will be boarding their dogs and cats as the holidays approach. I would not be surprised if we see some canine H1N1v infections confirmed, nor would I be surprised if we see a dual infection or two.
This pets-with-swine flu development does potentially complicate things. It means that surveillance needs to take on additional forms, particularly overseas in Asia where dual H5/H1v infections could occur. It means additional "mixing vessels" where avian flu and swine flu could reassort and emerge, infecting pigs, birds and, potentially, people. Surveillance has failed us once this decade, as public and animal health experts were so focused on swabbing birds' asses in the search for H5 that they missed the H1 swine flu pandemic that was brewing right under their noses. What makes matters worse is the grossly underreported fact that swine flu happens in this nation all the time.
So let's alert the vets of America to this new wrinkle, and let's do a little surveillance to see if cats and dogs are bringing their masters more than just dead birds and old shoes.
Why telecommuting will probably fail in a pandemic, Vol. 6: the GAO weighs in
 Dear reader,
Dear reader,
When I say you can get bleeding-edge commentary at this blogsite, I ain't whistling Dixie! As you know if you have been following this Blogsite for any length of time, I have an ongoing series about telecommuting and pandemics. I lecture on the topic frequently, and my last lecture on the topic was at the recent CIDRAP Summit in Minneapolis. The consensus global experts on this issue are Ken McGee of Gartner and myself. I have spent more than three years working on this issue, and my pessimistic conclusions can best be summed up in my latest Powerpoint presentation, Will Telework Work? Keeping Information Technology Up If People Go Down. A slide from that presentation is above.
That presentation, which is also available via subscription at the CIDRAP Business Source Website (along with all the other presentations from that event), serves as both a blueprint for erecting a work-from-home program as well as a cautionary tale about putting too much reliance in telework as a truly viable means of getting work done while keeping oneself (or hisownself, as Joe R. Lansdale would say) out of the office.
Now, the federal Government Accountability Office(HA! What an oxymoron!) has produced a document which pretty much parrots everything I have been saying for the past three years. First, please go back and search this Blogsite for the keyword "telecommuting" and read my prior blogs on the topic. I'll wait.
Back so soon?! Most excellent. Now let us look at the GAO report and analyze it. Sure enough, it mentions everything we have been talking about. Namely, Junior playing XBoxLive while Mom and Dad are trying to access the corporate mainframe. All this, from home cable and DSL routers that are not nearly as fault-tolerant as the T-1 and T-3 connections that are the norm for corporate offices.
You see, cable is the "party line" of the Internet. A cable modem connection is not a "home run" back to the local telco office that a T-1 or T-3 is. Sure, at night you might run a 2wire.com speed test and get 6 megabits of throughput, but that is at night, when things are quieter, Internet-wise. But as connections become more frequent, and the multiple demands on bandwidth accelerate, that cable bandwidth gets divided -- and devoured -- in a hurry. And you are sharing that cable Internet pipeline with everyone else in your neighborhood and adjacent neighborhoods who also have cable.
DSL is supposed to be a "home run," or direct cable run back to the CO, or Central Office of the telco, but from my own personal experience I am not sure that is truly so. DSL has made some huge strides, including the configuration of special DSL repeaters, in order to extend its availability beyond the 10,000 feet or so from the Central Office that was the original limit of DSL technology.
But those repeaters come with a significant gradual performance hit -- a loss of bandwidth the further out you get from the CO. For that reason, and because these repeaters are shared with other users, I cannot say DSL is a "home run" back to the telco. For those of you who want to "geek out" on a Wikipedia chart illustrating this DSL issue, here ya go!
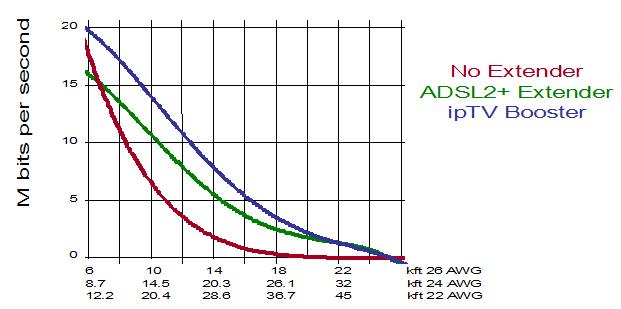
See, excitement abounds here!
Back to the issue at hand. Cable and DSL are consumer technologies, and are not nearly as reliable as good old-fashioned T-1 lines. You cannot as a general rule purchase priority restoration for cable or DSL technologies, meaning in a natural disaster these solution providers are the "last ones up the pole" to fix problems, after the electric utility and the traditional telco. So forget about cable or DSL robustness when it comes to these consumer solutions -- especially during hurricane season, or during winter in the frigid North. Or during a pandemic, when their (younger) workforce will be as strapped as yours.
There are a few quotes from the GAO study that need to be highlighted. First, from page i:
Increased demand during a severe pandemic could exceed the capacities of Internet providers’ access networks for residential users and interfere with teleworkers in the securities market and other sectors, according to a DHS study and providers (see figure below). Private Internet providers have limited ability to prioritize traffic or take other actions that could assist critical teleworkers. Some actions, such as reducing customers’ transmission speeds or blocking popular Web sites, could negatively impact e-commerce and require government authorization. However, DHS has not developed a strategy to address potential Internet congestion or worked with federal partners to ensure that sufficient authorities to act exist. It also has not assessed the feasibility of conducting a campaign to obtain public cooperation to reduce nonessential Internet use to relieve congestion. DHS also has not begun coordinating with other federal and private sector entities to assess other actions that could be taken or determine what authorities may be needed to act.
Next, from pages 14-16:
Increased use of the Internet by students, teleworkers, and others during a severe pandemic is expected to create congestion in Internet access networks that serve metropolitan and other residential neighborhoods. For example, localities may choose to close schools and these students, confined at home, will likely look to the Internet for entertainment, including downloading or “streaming” videos, playing online games, and engaging in potential activities that may consume large amounts of network capacity (bandwidth). Additionally, people who are ill or are caring for sick family members will be at home and could add to Internet traffic by accessing online sites for health, news, and other information. This increased and sustained recreational or other use by the general public during a pandemic outbreak will likely lead to a significant increase in traffic on residential networks. If theaters, sporting events, or other public gatherings are curtailed, use of the Internet for entertainment and information is likely to increase even more. Furthermore, the government has recommended teleworking as an option for businesses to keep operations running during a pandemic. Thus, many workers will be working from home, competing with recreational and other users for bandwidth.
During a pandemic, congestion is most likely to occur in the traffic to or from the aggregation devices that serve residential neighborhoods, interfering with teleworkers’ and others’ ability to use the Internet.
Congestion affecting home users is likely to occur because the parts of providers’ DSL, cable, satellite, and other types of networks that provide access to the Internet from residential neighborhoods are not designed to carry all the potential traffic that users could generate in a particular neighborhood or that all connect to a particular aggregating device for efficiency and cost reasons. Providers do not build networks to handle 100 percent of the total traffic that could be generated because users are neither active on the network all at the same time, nor are they sending maximum traffic at all times.
I will spare you the rest, which is basically a call to action on behalf of DHS to figure out how it can parcel out bandwidth in a pandemic. Good luck with that one! Who is to say that a teleworker for a state department of emergency management cannot get priority access because s/he is simply on a DSL or cable modem from home?
Dirty Little Secret: Key government operatives carry a card with them. This card guarantees them dial tone, if they can achieve dial tone. Once they have dial tone, a call to the secret number gives them priority access to the phone grid.
Dirty Little Secret 2: If you do not have dial tone, do not hang up the phone. Eventually, if there is dial tone to be had, you will get dial tone. Hanging up actually means you'll lose out in the hunt for dial tone.
So maybe we need an Internet Access Priority Card. Or a priority encryption scheme, or a priority Verisign authentication keyfob (there, that'll work). If you are a government or critical infrastructure type, you could plug in this USB thumbdrive with the appropriate levels of security, encryption and authentication, and you could merrily compute while your neighbors stew over speeds reminiscent of 1200 baud modems. If you don't know the word "baud," you are a pup.
The report is 77 pages, and is worth the read if you ever thought you could just send employees home with a laptop and keep the business humming. then go over to the link and download my Powerpoint on the topic, and get smart quick.
Nice to see the government agreeing with my take on things.
Underachieving virus, mixed Federal messages contribute to H1N1 swine flu vaccine debacle
In yesterday's blog, I mentioned what I thought were the root causes of the H1N1v vaccine shortage/delay situation.
So as I always try to do, I reached out to an expert to get his opinion and bounce theories off of him. In this case, the expert is one of the top scientists in the field of vaccine research: Dr. Greg Poland of the Mayo Clinic.
His introductory paragraph from the Mayo Clinic Website states:
 Vaccine-preventable infectious diseases; vaccines against agents of bioterrorism; predictors of vaccine response; antigen processing and HLA presentation; vaccine immunogenetics; cancer vaccines.
Vaccine-preventable infectious diseases; vaccines against agents of bioterrorism; predictors of vaccine response; antigen processing and HLA presentation; vaccine immunogenetics; cancer vaccines.
I have been seeking Dr. Poland's counsel for several years, and he has always proven himself to be receptive, affable, and eager to contribute. He is, in short, a great guy, though I have never met him. I feel as though I have, and one day I shall.
Anyway, Dr. Poland concurred with my belief that the two major factors that have contributed to the delays are a low-yield, difficult-to-grow virus; plus an "overly optimistic" Federal government spin on the vaccine situation.
While the early stages of this pandemic were managed according to the Bush playbook, authored by then-CDC head Dr. Julie Gerberding and then-HHS Secretary Mike Leavitt, this is now Obama's pandemic to manage. And the setbacks and mixed risk communications are becoming apparent.
The vaccine is no longer "early." In fact, by this time in Florida alone, there should have been some 3.787 million doses of H1N1 vaccine shipped to providers in-state. That is what was promised to the State of Florida back in September. Most of those shots would have gone directly to the "FloridaShots" network of 3,000+ health care providers and counth health departments.
But instead, Florida has only received some 900,000 doses of vaccine, a combination of FluMist and injectable vaccine. This is not even 25% of the doses promised by the Feds.
Likewise, back in September, the Feds had told the states there would be some 63.8 million doses of vaccine available by the end of October. Now, Washington is saying they will be lucky to deliver 28 million doses, or roughly 44% of the originally-promised total.
What, you say? the Feds promised 63.8 million doses? The media are reporting it was only 40 million! Wrong, swine breath. The original early September figure for planning purposes was 63.8 million doses nationwide. The Feds revised that 63.8 million figure downward to 40 million, then downward again to 28 million.
And in order for Washington to even keep that promise, it would have to send Florida 780,000 doses of vaccine within the next three days. Now how likely is that? I am not holding my breath. Nor should you.
Florida, instead of being promised 3.787 million doses of vaccine by the end of October, is now promised 5 million doses by the end of December.
 State and county health departments have been working 24/7 since August, planning and planning and planning some more for clinics that have been cancelled, postponed, rescheduled and then postponed yet again as the vaccine numbers slip. This is absolutely not their fault. Fortunately, some vaccine clinics have been successful, if running out of vaccine within 70 minutes (like they did in Baltimore recently, see photo above) can be considered successful.
State and county health departments have been working 24/7 since August, planning and planning and planning some more for clinics that have been cancelled, postponed, rescheduled and then postponed yet again as the vaccine numbers slip. This is absolutely not their fault. Fortunately, some vaccine clinics have been successful, if running out of vaccine within 70 minutes (like they did in Baltimore recently, see photo above) can be considered successful.
State and county health departments are tired, frustrated and just want consistent information that they can use to make sound plans. They do not need inaccurate promises of vaccine deliveries to make their dire situations even worse.
And in some cities, such as Los Angeles, health authorities are catching flak for inoculating anyone who showed up -- even if they were not in the target groups for vaccine. What is equally interesting about the LA experience is that the private providers are all out of vaccine, and the free clinics are the only ones with vaccine to give. So the persons in the target groups who had been waiting for their private doctor to administer the shot found out they were out of luck, so they opted for the public option, so to speak. It asks the question about whether or not the distribution of vaccine amongst private and public providers, especially in California, is an equitable and fair one.
So let's recap: We have insufficient vaccine, which is the fault of the virus itself for not growing rapidly enough in labs to produce sufficient amounts of vaccine. Damned underachieving virus! Additionally, the seed stock of virus, even after being turbocharged by the CDC and others, simply did not respond well enough to grow quickly.
The Federal government knew this back in the summer, and that was dutifully reported here at this blogsite and in the blogosphere and sometimes in the mainstream media. Nonetheless, when the vaccine makers said they could deliver vaccine earlier than expected, the Administration chose to trumpet this to the press, which dutifully reported it to the American people (and as I did and others in the blogosphere). Unfortunately, the Administration did not choose to "trust but verify," as a great president once recommended, and now it is trying to distance itself from a problem of its own exacerbation by making claims, as HHS Secretary Sibelius said this week, that there would be "an ample supply" of vaccine.
Really? When?
Is it any wonder that the Obama Administration just declared a national emergency over swine flu? Perhaps they are reading the tea leaves, as I am reading them. Here's what I see.
We are going to have a nasty winter. No, we're going to have a milder winter. That depends on what our old buddy El Nino does. For the mid-Atlantic states south, it means colder and wetter winter. Imagine a line from northern Virginia west to the Texas Panhandle, south to Lubbockand southeast to Key West. Cold, wet and generally crappy winter is forecast within that area. Maybe the rest of the nation, too, if the El Nino is weak, as some are beginning to forecast.
Couple this coming winter's uncertainty with the absolute certainty that vaccine will be in short supply until Christmastime, and you have perfect conditions for a return of H1N1v as soon as temperatures drop to regular winter levels. The sad fact is that we have lost the ability to vaccinate in sufficient numbers in time for the arrival of winter.
Add in the fact that people outside of the target groups are demanding and getting vaccine, means that those who really need it may be forced to stand in line and wait. And wait.
The Administration's declaration opens up the financial pipeline for Federal reimbursement for costs of triage centers set up far away from the hospitals that they might be connected to. That tells me that this Administration thinks things will get much, much worse before they get better; that the prevailing (yet hushed) talk in DC and elsewhere is of a virus that is poised to strike like a rattler once temperatures stay low and the heart of winter sets in.
Now add in the shortage of vaccine, and you have what many of us have predicted for years: A flu pandemic where vaccine was not readily available until well into the second wave of the pandemic.
Why anyone would have not stayed "on message" regarding this vaccine scenario is beyond me. As Karl Malden's Omar Bradley said to George C. Scott's Patton: "Well, I would give myself a little leeway if I were you."
A new question I now have is this: Can we still trust that one shot will provide sufficient protection? After all, the virus is growing slowly and underperforming. There is tremendous pressure to deliver vaccine at any cost. Are we testing to ensure the vaccine is as potent as promised?
Anyone have an answer?
Swine flu, Obama, vaccine shortages, BioCryst, national emergencies and a feeling of unease
First off, let me apologize for my lack of blogs the past FIVE WEEKS. As I always say, however, I will not blog if I do not have something to say. There are others (Mike Coston, Crof, Revere) who get the job done day in and day out, and so I leave them to do their thing.
I have actually had plenty to say recently, but I flat out have not had the time to write. So let me catch you all up on events in the McPherson household:
Wife is recovering from her chemo. Daughter is fine and long-over The Swine. However, son came down with H1N1 at his school in Long island, Stony Brook (Go Seawolves!). I might add, they are 4-4 and are battling for the conference title, and my boy is contributing nicely as a D-lineman.
Fortunately, I had delivered some Tamiflu for him (and his girlfriend) on our last visit. Knocked it out of him within 36 hours. Tamiflu is really amazing. He also did the "double dose" on the first treatment, and I am more convinced than ever that this is an excellent way to go. TomDVM agrees, as you might have read on a previous comment.
Work has also conspired to keep me occupied. So now you are caught up. However, within the past ten days there have been several news stories which I will attempt to take on with a sweeping (rambling?) monologue.
First, the issue of H1N1 vaccine shortages. Why is everyone so worked up about these manufacturing delays? Because someone(s) set the bar too high to begin with. May I refer back to my previous blogs regarding the delays experienced by vaccine manufacturers in the late spring. The seed stock was not efficient enough, and the virus was growing ever-so-slowly in eggs. So the CDC, or the WHO, or whomever does these things, grew new seed stock and sent it out to the vaccine makers (all six of them, or however many there still be).
What is apparent from these delays is that the problem of slow-growing virus never truly went away. And it would be very interesting to know exactly why the vaccine is moving so slowly down the pipeline. Was the virus drifting during manufacture? Was there just not enough yield? Was the virus killing the eggs (which we have seen in H5N1 vaccine manufacturing)? If the latter is true, then that is a cause for future concern.
Or were some policy-spinners in DC just too damn quick with promises of ample vaccine in the month of October?
One thing is for sure: State and county health departments are having enormous difficulties in scheduling mass vaccination programs based on delivery assurances from Washington that are ringing hollow.
Which leads us to the weekend declaration from President Obama that H1N1v has become a National Emergency. Now before everyone gets too worked up about this, be assured that sometimes one has to move to this level in order to circumvent certain existing rules and policies that can combine to slow the treatment of patients.
The declaration does several things. First, it allows more flexibility in federal reimbursements for triage centers that may be set up hundreds of yards away from the hospital property, say in an elementary school cafetorium. Second, it allows more rapid movement of people and stuff. And third, it does re-focus attention on the existing problem of H1N1v.
So don't make too much, nor too little, about the presidential declaration.
Now on to the BioCryst antiviral, peramivir. Loyal readers of this Blogsite have followed my blogs about this Birmingham, Alabama-based company for years. And it seems that, finally, it is positioned at the right place in the right time. The FDA has given its approval for the emergency use of its injectable antiviral peramivir to very seriously ill H1N1 patients. And none too soon, as both H1N1v cases and deaths continue to increase. If they can keep enough medicine in the pipeline, we could have a very powerful weapon in the flu arsenal.
Now for my feeling of unease. Here in sunny Tallahassee, we have seen a spike in the number of flu cases, followed by a lull, and then another recent spike. In Florida, we are seeing an average of ten deaths per week directly attributable to "lab-confirmed" swine flu. Who knows how many other deaths we have missed; that will be worked out by the statisticians later on.
But I just don't feel like we have seen everything this virus can deliver yet. I am looking at the latest Florida statistics as I write this. Leon County (Tallahassee) is currently not reporting widespread flu activity, a trend that seems to be repeated throughout the urban areas. In the meantime, the virus is considered widespread in southwest Florida, a haven for retireds and winter residents.
What really distresses me are the Florida flu map's shaded areas that signify "no report." There are about a dozen "serial non-reporters," county health departments who have not reported their status the last two weeks in a row. Most of these are in the Panhandle and the "Big Bend" area that joins the Panhandle to the peninsula. Don't they know there's a pandemic on? If they are not reporting their status, how do we know if they have recorded any deaths attributable to flu?
Rant over; now back to the issue at hand. I am likening my feeling to the calm that precedes the storm. This virus, I believe, is still far from having finished seeding itself across the globe. Nor do I believe the virus has finished its first wave across Asia. It moved so quickly across the industrialized world, then resumed its regular pace (or has seemed to me to do so) as it crossed those areas where transportation is basically unchanged from what it was fifty years ago.
For much of the developing world, the first "seed" wave is still upon them, even as we move into what will certainly be known as the pandemic's second wave in the Americas. Just how long it will take for this virus to emerge from Asia, and what form it will take once it does so, is the subject of much speculation.
And while schoolchildren are understandably getting a lot of attention as victims of this virus, here in Florida, look at the faces of death over the past three weeks:
A 20-year-old female in Alachua County, a 52-year-old female in Baker County, a 24-year-old-female in Citrus County, a 49-year-old female in Miami-Dade County, a 78-year-old male in Miami-Dade County, a 53-year-old female in Hernando County, and a 15-year-old male in Volusia County. A 49-year-old female in Broward County, a 49-year-old female in Miami-Dade County, a 67-year-old female in Miami-Dade County, a 50-year-old female in Duval County, a 47-year-old male in Lake County, a 37-year-old female in Manatee County, a 35-year-old male in Pasco County, a 43-year-old female in Pinellas County,
a 50-year-old female in Pinellas County, a 56-year-old female in Polk County, a 58-year-old male in Volusia County, and a 10-month-old female in Sarasota County. a 51-year-old male in Brevard County, a 55-year-old female in Charlotte County, a 62-year old female in Desoto County, a 51-year-old female in Hillsborough County, a 30-year old female in Lee County, a 55-year-old male in Monroe County, a 33-year-old female in Okaloosa County, a 45-year-old male in Pasco County, a 64-year-old female in
Pinellas County, and a 45-year-old male in St. John’s County.
That is an average age at death of 46 years. It will be interesting to see just how far upward the average age at death is trending nationwide. But in Florida, where the average living age is older than most states, it appears as though the virus is taking a wider swath of victims -- and what that may portend for the coming months is unsettling.
Intranasal and Novartis H1N1 vaccines still not for egg-sensitive people
Recently, I was sitting in my dentist's chair when an assistant remarked she could not receive flu vaccine due to her allergy to eggs and egg products.
I listened intently and replied,
"Cauuuuuuggggggwwwwwllllllhhhhhh. MMmmmugggggggwwwaaaaaawwwwaaahhh."
After the assistant removed all the stuff in my mouth, I then translated: I would check this out and get back to her.
Well, I happened upon the insert to the monovalent H1N1v vaccine manufactured by Medimmune. Medimmune manufactures a live virus, intranasal vaccine principally for youth, and they are one of the four FDA-approved pandemic vaccines.
But I noticed this entry:
4 CONTRAINDICATIONS
4.1 Hypersensitivity
Influenza A (H1N1) 2009 Monovalent Vaccine Live, Intranasal is contraindicated in individuals with a history of hypersensitivity, especially anaphylactic reactions, to eggs, egg proteins, gentamicin, gelatin, or arginine or with life-threatening reactions to previous influenza vaccinations.
Likewise, I also saw this factoid:
------------------------------DRUG INTERACTIONS-------------------------------
• Antiviral agents active against influenza A and/or B: Do not administer Influenza A (H1N1) 2009 Monovalent Vaccine Live, Intranasal until 48 hours after antiviral cessation. Antiviral agents should not be administered until 2 weeks after Influenza A (H1N1) 2009 Monovalent Vaccine Live, Intranasal administration unless medically necessary.
(7.2)
So, you have to be off Tamiflu and Relenza for at least two days prior to accepting this vaccine into your schnozz. Probably because FluMist is live virus, and the antivirals might kill the virus before it helps you.
Finally, immunocompromised persons should not receive the vaccine intranasally.
5.4 Altered Immunocompetence
Administration of Influenza A (H1N1) 2009 Monovalent Vaccine Live, Intranasal, or FluMist live virus vaccine, to immunocompromised persons should be based on careful consideration of potential benefits and risks. Although FluMist was studied in 57 asymptomatic or mildly symptomatic adults with HIV infection [see Clinical Studies (14.3)], data supporting the safety and effectiveness of FluMist administration in immunocompromised individuals are limited.
I had never realized that FluMist was not for persons with sensitivity to eggs. So in the event others thought as I did (or did not), this is offered as a helpful; and potentially lifesaving reminder.
Buit wait, you say! Wasn't there word Novartis was making a vaccine from cells, rather than eggs, and they had whipped up a whopping ten liters of vaccine?
That's what the media ballyhooed. But again, directly from the Novartis package insert:
CONTRAINDICATIONS
•
History of systemic hypersensitivity reactions to egg proteins, or any other component of Influenza A (H1N1) 2009 Monovalent Vaccine, or life-threatening reactions to previous influenza vaccinations. (4, 11)
So there you go. None of the four vaccines can be taken by persons sensitive to eggs. I am unsure how much of the US population falls into that category, but I am betting it is a significant number. Better save the antivirals for them! Easpecially persons with egg allergies and under age 50.
Scott
PS. I will try to blog from the CIDRAP Summit in Minneapolis. Mostly, I hope FLA_MEDIC and I get around to some used book and comic stores! FLA_MEDIC should be quite the celebrity after this is all said and done! He is appearing with Robert Bazell of NBC News, one of my favorite correspondents, and Katie Couric's producer. The print editors are no slouches, either.
I think I will tell Mike some Katie Couric stories when she was a cub reporter at Channel 4 in Miami and I ws running for office......
